Summary
Physical Description
Ecology
Symbiosis
Life History & Behavior
Feeding Behavior
Movement
Reproduction and Development
Respiration
Response to Light Changes
Anatomy & Physiology
Circulatory and Excretory Systems
Defense Mechanisms: Cnidocytes and Cnidae
Digestive System
Nervous and Sensory Systems
Skeleton and Musculature
Evolution and Systematics
Biogeographic Distribution
Conservation and Threats
References and Links | Defense Mechanisms: Cnidocytes and Cnidae
Animals from the phylum Cnidaria are characterized by the presence of the cnidocyte, which is a combined sensory-effector cell that houses a subcellular fluid-filled membranous organelle known as a cnida1,2. Upon receiving the right combination of mechanical and chemical stimulation, cnidocytes may evert the long tubular invagination of its capsule wall explosively to the exterior. They tend to be aggregated in higher numbers on the tentacles, other prey-catching or defensive structures or near the mouth1.
There are three types of cnidae: nematocysts, spirocysts and ptychocysts, and they are housed in three types of cnidocytes: nematocytes, spirocytes and ptychocytes respectively. All three types of cnidocytes can be found in sea anemones. In sea anemones, the nematocytes have a motile sensory cilium that is mechanoreceptive known as a ciliary cone and the firing site may be covered by three apical flaps. The nematocysts within them have a thick wall and often have spines or barbs on the surface of the everted tubule1. If toxic, they can contain a high concentration of polypeptides and proteins that act as neurotoxins, hemolysins and enzymes, and can cause cardiotoxicity, dermatitis, local itching, swelling, erythema, paralysis, pain and necrosis in humans3. Toxins are injected into prey or predators when the mechanoreceptive sensory cilium receives the appropriate stimulation1. They function in prey acquisition, predator deterrence and intraspecies spatial competition4.

|
| Figure 1. Different types of nematocysts, everted (right) and uneverted (left). Adapted from Shick (1991). |
There are two broad categories of nematocysts: haplonemes and heteronemes. Neither the tube nor its spiny armature (if present) is divided into noticeable regions in haplonemes. Haplonemes include atrichs, which lack spines, and holotrichs, which are equipped with spines that usually occur along the full length of the tube. Holotrichs can be isorhizous or anisorhizous, the latter of which has a gradual but obvious change in diameter or spination along its length.
Conversely, heteronemes always have spines. The thread is separated from the tube that has an obvious basal shaft by a greater diameter, large spines, or both. They can occur as either p-mastigophores or b-mastigophores. The shaft of p-mastigophores is always thicker than the thread (which may or may not have spines) and the two meet at a characteristic cone-shaped invagination into the shaft5. P-mastigophores are penetrants used in prey capture6, and can be macrobasic and microbasic. Macrobasic p-mastigophores have a longer undischarged shaft than the capsule, hence the shaft is folded inside the capsule. On the other hand, the shaft of microbasic p-mastigophores is shorter than the capsule, hence it is unfolded. There are also amastigophores, which are macrobasic p-mastigophores but with small terminal threads that may break off and remain inside the capsule after discharge. B-mastigophores are also known as basitrichs. When uneverted, the shaft lies straight within the capsule and tapers into the thread that typically coils evenly around the shaft, and always bears spines5 (Figure 1).
Spirocysts on the other hand are thin-walled. Its undischarged tubule is coiled like a spring and lacks barbs or spines, but has adhesive and minute threads that radiate from the wall of the tubule, hence it has adhesive properties1,2.
As for ptychocysts, which are unique to anemones from the order Ceriantharia, they serve more of a protective function2. Although the ptychocyst also contains an adhesive tubule, it lacks the radiating sticky threads that the spirocyst has. Moreover, the tubule is folded in a compact zigzag manner within the capsule1.
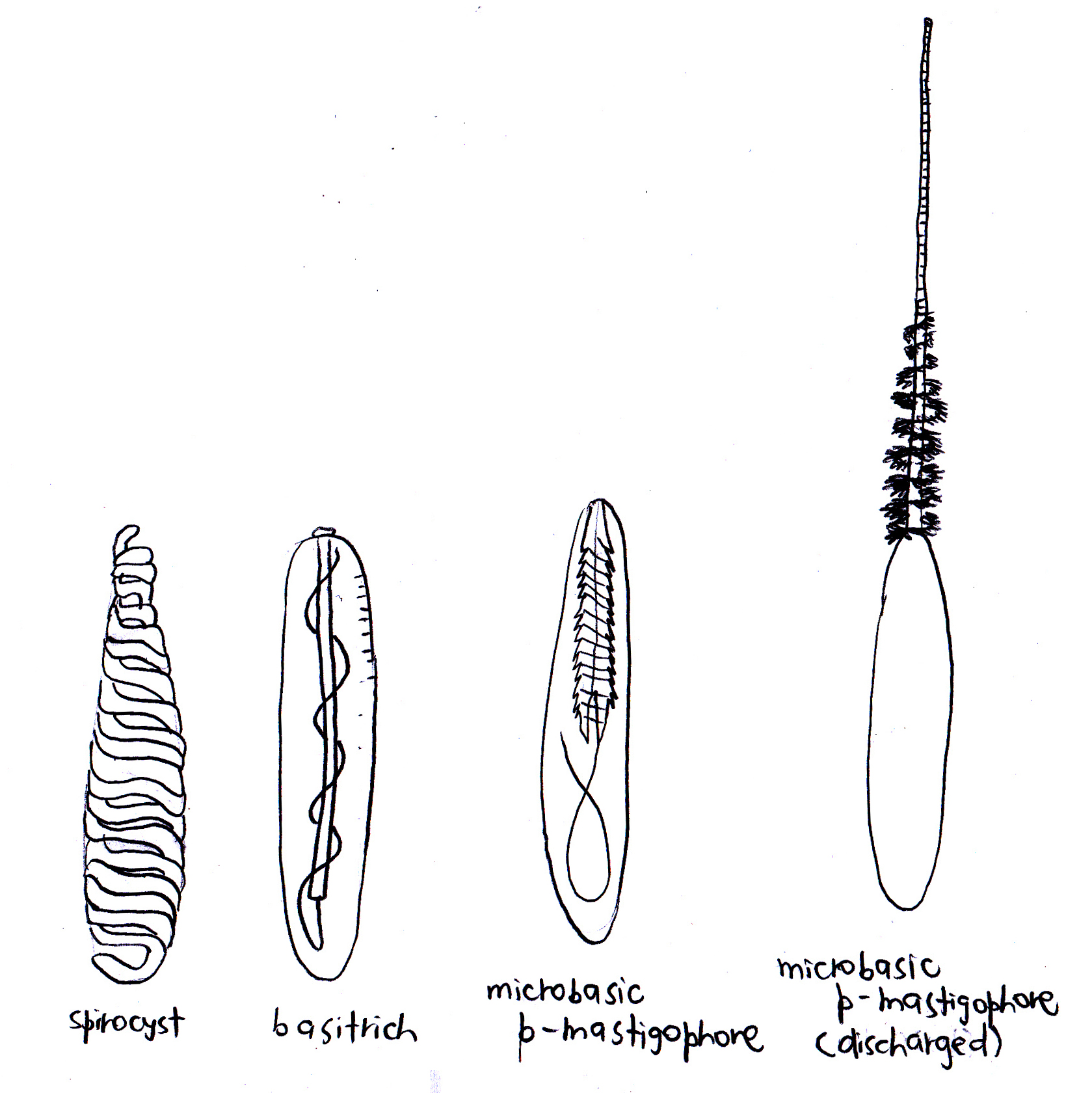 |
Figure 2. Cnidae known to be found in C. adhaesivum. Adapted from Carlgren (1940), Dunn (1981) and Hutchings, Kingsford & Hoegh-Guldberg (2008).
|
Not only do actiniarians have one of the most varied diversity in terms of their cnidae7, individuals may also have their own characteristic cnidome or size distribution of cnidae on a particular region or structure on their bodies8. In the case of Cryptodendrum adhaesivum, it is known to have a powerful sting9,10,11 and can damage sensitive parts of the human skin9. Three types of cnidae have been observed in C. adhaesivum: basitrichs, microbasic p-mastigophores, and spirocysts (Figure 2).
The cnidae type and the respective locations at which they have been observed to occur are listed below:
|
Location
|
Cnidae type
|
Range of length (µm)
|
|
Actinopharynx
|
Basitrichs
|
26—32.5
|
|
11.5—20.5
|
|
26.2—33.6
|
|
Microbasic p-mastigophores
|
41–
|
|
Spirocysts
|
20.5—27.9
|
|
Column
|
Basitrichs
|
20.5—24
|
|
20—26.3
|
|
38.5—41.8
|
|
Filaments
|
Basitrichs
|
11.5—14
|
|
25.5—32
|
|
11.5—13.9
|
|
25.4—38.5
|
|
Microbasic p-mastigophores
|
32.5—38
|
|
37.7—42.6
|
|
Nematospheres
|
Basitrichs
|
34.4—40.2
|
|
Spirocysts
|
15—31.2
|
|
Tentacles
|
Basitrichs
|
17—19
|
|
32.5—37.5
|
|
17—24
|
|
9.8—11.5
|
|
17.2—26.2
|
|
34.4—40.2
|
|
Microbasic p-mastigophores
|
37—38
|
|
Spirocysts
|
15—20.5
|
|
17.5—33.6
|
(as observed by Carlgren 1940; Dunn 1981)
The bulbous tentacles of the C. adhaesivum individual found on Heron Island were observed to have numerous cnidae under a light microscope (saggital section through tentacles; Figure 3, 4, 5).
.png)
Figure 3. Cnidae in bulbous tentacles of Cryptodendrum adhaesivum (x100).
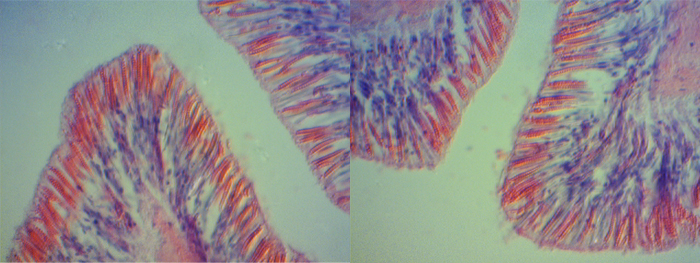
Figure 4. Close-up of cnidae in bulbous tentacles of Cryptodendrum adhaesivum.
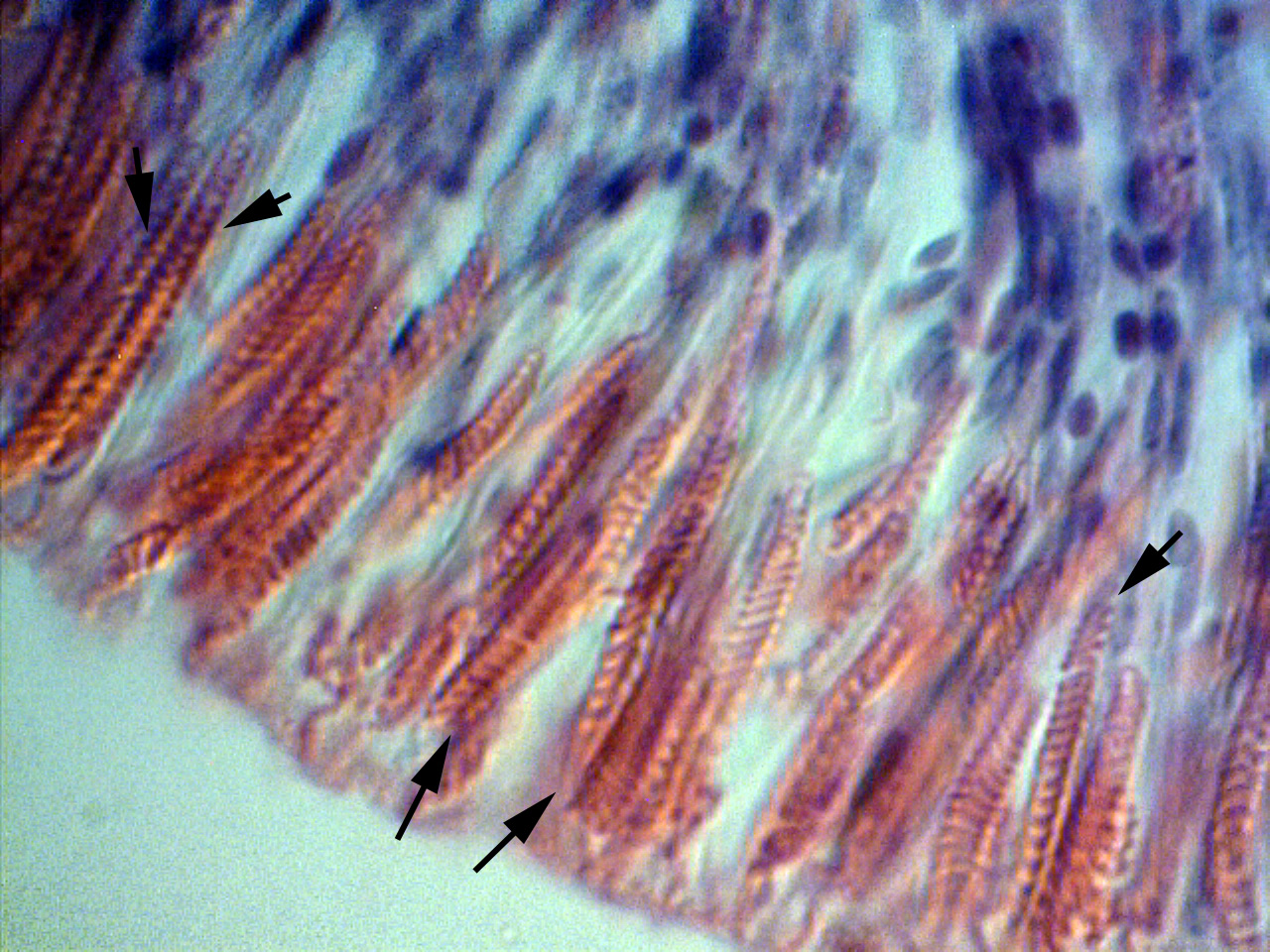
Figure 5. A closer look at the sagittal section of a bulbous tentacle of Cryptodendrum adhaesivum indicates they are spirocysts.
A range of different compounds have been noted to be found in cnidocytes, and approximately 250 compounds are currently identified. They include peptides, proteins, enzymes, proteinase inhibitors and non-proteinaceous substances such as purines, quaternary ammonium compounds, biogenic amines and betaines. However, the exact details of the venom composition still remain largely unknown, and very few toxin-encoding genes and protein three-dimensional structures have been described. Sea anemone toxins are primarily made up of cytolytic or neurotoxic proteins and peptides with a varying potency depending on the structure and site of action. Neurotoxins cause convulsions and immobilization in prey by affecting Na+ channels; they slow the rate of Na+ channel inactivation, hence lengthening the repolarization phase in excitable membranes and subsequently leads to a massive release of neurotransmitter that paralyzes prey8. Examples of other toxins include acid-sensing ion channel toxins, Cytolysins, toxins with Kunitz-type protease inhibitors activity or with Phospholipase A2 activity, and voltage-gated Na+ and K+ channels toxins2. Different families have been noted to contain either type 1 sodium channel toxins, type 2 sodium channel toxins, or both. In C. adhaesivum, type 2 sodium channel peptide toxins have been found (δ-TLTX-Ca1a)12.
1Ruppert, Fox & Barnes 2004
2Frazão, Vasconcelos & Antunes 2012
3Martins et al. 2009
4Nevalainen 2004
5Weill 1934
6Mariscal 1974b
7Schmidt 1974
8Shick 1991
9Erhardt & Knop 2005
10Sprung 2001
11Halstead 2000
12Maeda, Honma & Shiomi 2010
|
|